Biomedical Engineering’s Jindřich Henry Kopeček and Hematology Professor Douglas Sborov (Co-PIs) received a grant from Bioscientific Innovations from Cancer Engineering Partnership (BICEPS) for their project “Engineering Biparatopic Antibody-Peptide Conjugate Targeting CD47/CD38 to Treat Multiple Myeloma”
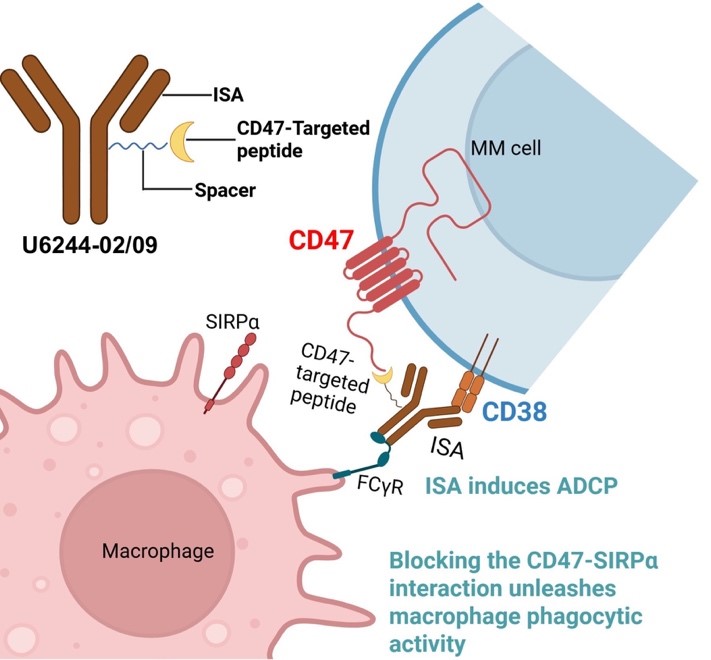
In the next fiscal year, they will evaluate the potential of innovative Antibody-peptide conjugates targeting CD47 and CD38 simultaneously to treat multiple myeloma (MM), a cancer that develops from a type of white blood cells in the bone marrow. MM remains largely incurable despite recent advances in targeted therapies and immunotherapies. CD38-targeting monoclonal antibodies (e.g., Isatuximab) have had improved outcomes, yet their efficacy is limited by immune evasion and antigen loss. Concurrently, CD47, a “don’t eat me” signal overexpressed on MM cells, enables tumor escape from phagocytosis via SIRPα (signal-regulatory protein alpha) binding. Although CD47 blockade enhances macrophage-mediated clearance, systemic inhibition has been hindered by hematologic toxicity due to its expression on normal cells. The construct combines Isatuximab with CMP22, a CD47/SIRPα-disrupting peptide, via a modular click chemistry–based platform. They hypothesize that the dual-targeting strategy will enhance tumor-selective immune activation by leveraging CD38-mediated targeting and spatially restricted CD47 blockade within the tumor microenvironment.
Illustration of the design of anti-CD38 mAb conjugated with a CD47-SIRPa blocking peptide.
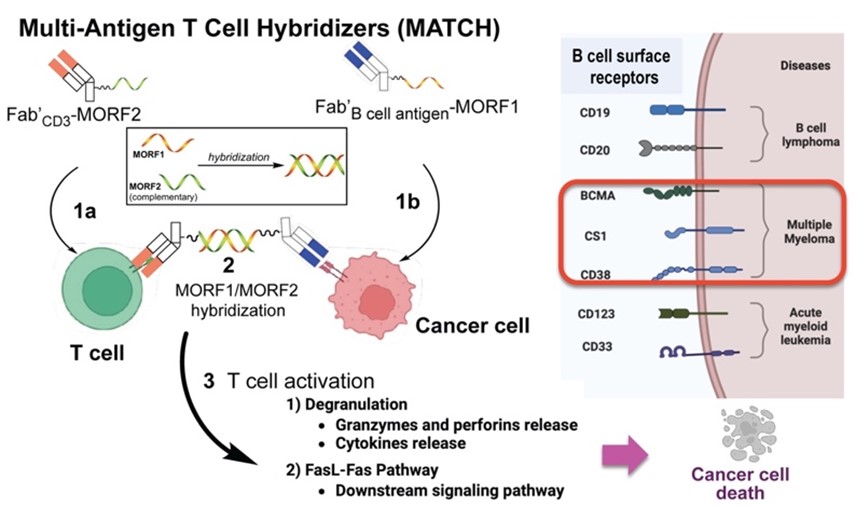
Multi-Antigen T Cell Hybridizers provide cancer cell specific T cell activation by utilizing a two-component system of complementary Fab’ motifs: i) Fab’CD3-MORF2 for T cell engagement, and ii) various Fab’B cell-MORF1 for cancer cell engagement. The two Fab’s then recognize each other to dimerize and complete the bispecific construct.
In addition, the competing renewal of the R01 grant “Drug-free macromolecular therapeutics (DFMT)” focusing on Multi-antigen T cell hybridizers (MATCH) for the treatment of multiple myeloma (J. Kopeček, J. Yang, MPIs) was evaluated in the INN Study Section and received 1 percentile ranking. In MATCH, a mini-library of cancer cell-targeting motifs, comprising antibody fragment Fab’-MORF1 conjugates (e.g., Fab’BCMA-MORF1, Fab’CD38-MORF1, Fab’SLAMF7-MORF1, Fab’CD20-MORF1, Fab’CD19-MORF1), selectively pairs with a T cell-engaging Fab’ conjugated to the complementary MORF2 (Fab’CD3-MORF2). The rapid, high-fidelity heterodimerization of complementary 25 base morpholino oligonucleotides (MORF 1/MORF2) enables the self-assembly of conjugates that function like bispecific antibodies. However, MATCH’s two-component “split-antibody” design offers a modular, customizable platform for designing bi- and multi-specific T cell recruitment therapies. Its key advantages include: (a) cell-specific targeting through interchangeable cancer cell-targeting motifs to address MM heterogeneity; (b) reduced cytokine release and T cell exhaustion; and (c) personalized therapeutic strategies.
The Kopeček/Yang Lab has made significant strides in their fight against multiple myeloma. Their contributions have been beneficial to cancer treatment improvements, and the Kopeček and Sborov grant will be crucial in continuing their research.