UBME Student, Shwan Javdan, Receives NIH F31 National Research Service Award (NRSA)!
My name is Shwan Javdan, and I am a Biomedical Engineering Ph.D. Candidate in the Deans Lab for Applied Synthetic Biology. I was born and raised in Salt Lake City, received dual B.S. degrees from the University of Utah in Biomedical Engineering and Chemistry, then spent a few years in Boston where I received my M.S. in Biomedical Engineering. In my free time, I enjoy skiing, board games with friends, and streaming great TV shows. The Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NRSA) program enables promising predoctoral students the opportunity to develop as independent research scientists, providing full stipend and tuition support for up to 3 years. In order to receive this award, I submitted a grant proposal to the NIH, totaling 52 pages, which included a personal biosketch, a sponsor statement, a detailed research proposal, my goals for fellowship training, and more. Additionally, several letters of recommendation were required.
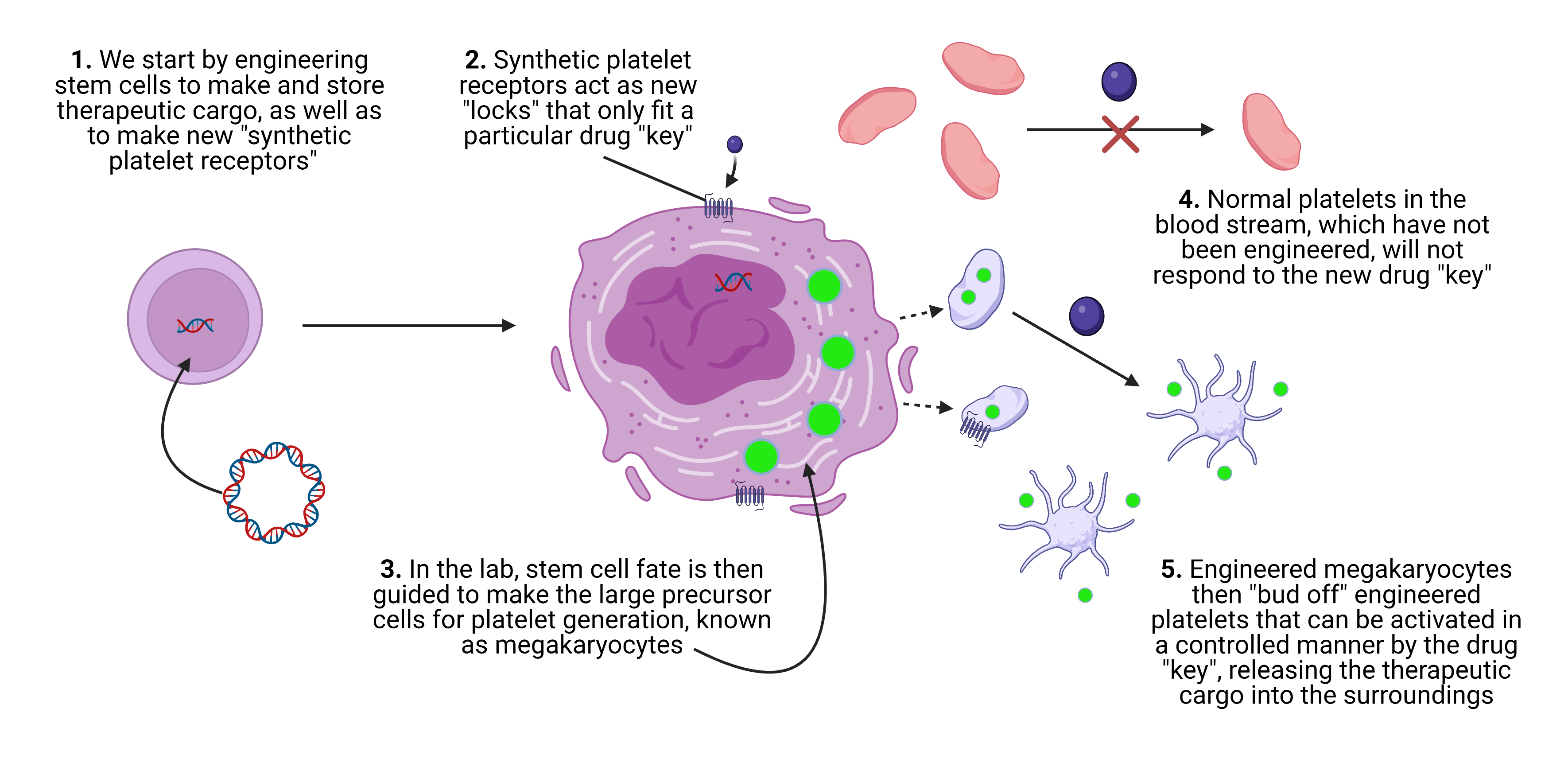
My research in The Deans lab focuses around applying synthetic biology tools to engineer cells with new functions. Specifically, I work on developing platelets as the next generation of cell-based therapies, taking advantage of their inherent biocompatibility and unique properties to store and deliver protein payloads. Normally, platelets are activated in the body by a protein called thrombin during injury. However, when engineering platelets in the lab with new functions, we wanted to be able to activate only the synthetic platelets we have introduced into the body in a controlled manner, without unintentionally activating the body’s regular platelets.
It turns out that if you can tap into the signaling pathway responsible for platelet activation and make some subtle changes, you can activate platelets with a simple small molecule drug. The analogy that I like to use is that of a lock and key. Platelets are covered in “locks” that fit thrombin, the “key” which activates them and causes them to release their protein cargo. By engineering a new “lock” on the surface of platelets, one that we know has a “key” that will not interact with other biological processes in the body, we can achieve precise control over only our synthetic platelets. This idea was the basis of my F31 research proposal, which ultimately lays the foundation for platelets as novel therapeutic delivery vehicles. We hope that one day, our synthetic platelet platform can be used to treat a host of diseases, including heart disease, lymphoma, and multiple myeloma.